FDA INSIGHTS NEWSLETTER
A newsletter of priceless wisdom from former FDA officials
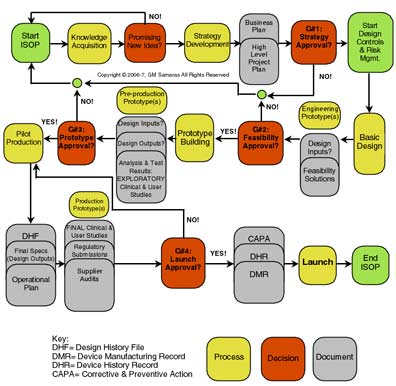
Samaras Flow Chart for Medical Device Development
George Samaras, Ph.D., DSc, PE, CPE, CQE
Former FDA Compliance Officer
Thinking About Developing a New Device?
There are so many steps to getting a great device idea from your head into production and ultimately reviewed and approved. The following flow chart was created by a former CDRH Compliance Officer, George M. Samaras, Ph.D., DSc, PE, CPE, CQE who is in the midst of publishing a text titled Human-Centered Product and Process Engineering: Safe, Effective, Efficient and Satisfying for Users
Each one of these steps requires countless hours of collaboration and expertise. This is precisely what our FDA consulting team can help you with. From concept to commerce, PCI is with you every step of the way.
Innovation Standard Operating Procedure (ISOP) for Regulated Medical Device Development under Design Controls (21 CFR 820.30 and ISO 13485:2003)
Click to see larger image of chart
See previous articles from our current issue of FDA Insights:
Top 10 Checklist for FDA Regulatory Submission
FDA Fights Battles on Two Critical Fronts |